 January 27, 2023
January 27, 2023
Concrete is one of the most widely used construction materials in the world due to its various advantages such as formability and durability. It serves as the foundation for much of civilization’s infrastructure and physical development. In fact, nearly twice as much concrete is used worldwide than all other building materials combined.
Concrete is a mixture of cement, sand, aggregate, and water, which is strong in compression but not in tension. This critical issue is addressed by incorporating reinforcing steel into the concrete and casting wet concrete around robust steel bars. When the concrete sets and hardens around the bars, we obtain a new composite material known as REINFORCED CONCRETE (RCC). This makes it a versatile material that works effectively in both compression and tension.
Concrete resists squeezing due to compressive strength, while steel resists bending and stretching due to tensile strength. This enhances ductility, reduces long-term deflections, or increases the flexural capacity for beams.
Corrosion of steel in concrete structures plays a significant role in affecting the service life of such structures. The corrosion of reinforcing steel can be a significant problem in concrete structures. The corrosion of steel produces hydrated iron oxide or rust, which is very detrimental and expensive. This expansion creates internal pressure, eventually leading to concrete failure in the form of spalling. This is a leading cause of concrete deterioration, and numerous studies have been performed to prevent the corrosion of reinforcing steel.
The first line of defense against reinforcement corrosion is inhibiting the penetration of water, oxygen, carbon dioxide, and salts from the concrete surface to the reinforcement. Several tests aim to assess permeability, diffusion, absorption, or other direct measures of fluid penetration resistance. The most frequently used test is ASTM C1202, commonly referred to as the Rapid Chloride Permeability Test (RCPT). The RCPT measures the electrical charge that travels between two sides of a concrete specimen during a six-hour period. This charge is correlated to chloride ions traveling through the pore system. Lower values indicate higher resistance to chloride intrusion.

Various methods have been developed with the intent of preventing corrosion and enhancing service life. These methods include coating the concrete surface, coating the reinforcement, cathodic protection, electrochemical methods, alternative reinforcement, and the use of corrosion inhibitors.
Corrosion is an electrochemical process, similar to that of a battery or fuel cell, but in reverse. There are both anodic and cathodic reactions involving the movement of electrons, resulting in the formation of corrosion products. In the anodic reaction, electrons are removed from the iron atoms in the steel, while in the cathodic reaction, those electrons are consumed.
Among all the available techniques, the use of corrosion inhibitors is one of the most appropriate and efficient methods for the corrosion protection of reinforced concrete structures due to its ease of operation, low cost, and excellent corrosion resistance effect.
The first line of defense against corrosion of reinforcement is to inhibit the penetration of water, oxygen, carbon dioxide, and salts from the concrete surface to the reinforcement. Quite a few tests try to assess permeability, diffusion, absorption, or other direct measures of fluid penetration resistance. The most frequently used is ASTM C1202. This test is commonly referred to as the Rapid Chloride Permeability Test (RCPT). The RCPT is a measurement of the electrical charge that travels between two sides of a concrete specimen during a six-hour period. This charge is correlated to chloride ions traveling through the pore system. Lower values signify a higher resistance to chloride intrusion.
Various methods have been developed with the intent of preventing corrosion and enhancing service life. The methods include the coating to the concrete surface, the coating to the reinforcement, cathodic protection, electrochemical methods, alternative reinforcement, and corrosion inhibitors.
Corrosion is an electrochemical process much like that of a battery or fuel cell, only in reverse. There are both anodic and cathodic reactions that involve the movement of electrons and result in the formation of corrosion products. In the anodic reaction, electrons are removed from the iron atoms in the steel, while in the cathodic reaction, those electrons are consumed.
Among all the available techniques, the use of corrosion inhibitors is one of the most appropriate and efficient methods for corrosion protection of reinforced concrete structures due to the easy operation, low cost, and excellent corrosion resistance effect.

MCON RASAYAN has introduced Bipolar Concrete Penetrating Corrosion Inhibiting Admixture MCON CI 300. It is added to concrete and seals steel reinforcement at both anodic & cathodic sites, for inhibition.
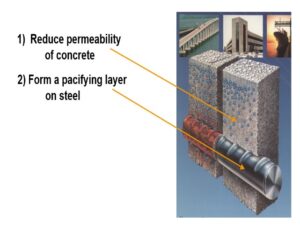
We need to protect the concrete structures from corrosion and the corrosion-inhibiting admixture is the right solution for the same. It plays the role of a vaccine for steel corrosion and is the perfect preventive measure needed for the protection of RCC.

Nandan Pradhan, a management degree holder and the Executive Director of MCON Rasayan, is the driving force behind the smooth operation of the organisation. His years of experience in planning, strategising, execution, team training, and ensuring productivity across all areas of company operations have made MCON Rasayan a global leader in the construction chemical manufacturing sector.




